
Healthy living
Use our expert advice and recommendations to live your best life every day.
Get started
The hype around pricey weight-loss injections has created huge demand for cheaper alternatives, but don't get drawn in by food supplements that claim to burn fat fast - we found many making claims that aren't allowed or backed by evidence.
Brands are claiming their products will help to burn fat, block carbs or suppress your appetite. Or, that their products can increase GLP-1 production (the appetite-regulating hormone that prescription jabs such as Wegovy and Mounjaro mimic), reduce cravings, and are even a ‘safe alternative’ to weight loss jabs.
But many of these claims are stretching the truth – or are based on little to no evidence – and brands shouldn't be making them.
We scoured more than 100 supplements from retailers ranging from high street health retailers to major online marketplaces and compared the claims made to what the listed ingredients are actually proven to do.
Our investigation uncovered lots of poor practice, including products with claims that aren't allowed, are unrealistic and lack any solid evidence of benefit. Some also have potential health risks.
In the UK, you aren't allowed to make health claims about a product without any evidence. If a business wants to advertise their product with a specific claim, it must either be on the approved list of claims relating to specific ingredients or the ‘on hold’ register.
The health claims register, managed by the Department of Health and Social Care (DHSC), shows what you can – and can’t – say an ingredient can do for your health, and the wording and conditions are often very specific.
‘On hold’ claims are neither approved nor rejected, but still allowed to be used whilst under consideration, provided the brand can justify the claim with its own high-quality evidence.
A government spokesperson told us: ‘Any claims about the health benefits or nutritional value of supplements need to be backed by science and officially approved by authorities. Companies that break these rules may be subject to enforcement action by local authorities.’
In practice, however, we found plenty of evidence that brands are getting away with making outlandish claims that are misleading consumers.

One of the most common claims was that supplements were ‘fat burners’.
Products with fat burner claims often included green tea extract, caffeine, or capsicum.
Dr Adam Collins, associate professor of nutrition at the University of Surrey, explains, ‘Some ingredients, like caffeine, green tea and capsicum, experimentally have been shown to work. But experimental evidence doesn't necessarily mean practical benefit. And although there might be some effect, it's not going to be as big an effect as you think.’
Claims for green tea and capsicum relating to weight loss and fat burning are ‘on hold’. This means they have been neither approved nor rejected, so there is no accepted effect of these ingredients. Claims that caffeine can reduce body weight have been rejected for use in marketing in the UK, because the evidence is not good enough.
There are some risks to ingesting too much of these ingredients, as excessive caffeine can lead to anxiety, sleeplessness and diarrhoea, while cases of liver damage have been linked to excessive green tea supplement consumption.
Dr Collins adds, ‘It's also not necessarily that the product has been formulated and then tested for effect on fat burning, it's just claiming that the ingredients included in that supplement have themselves been shown to increase fat oxidation [where the body breaks down fatty acids].’
But in the hundreds of products we looked at, claims were almost always made about the product as a whole.
Another common claim was that supplements could suppress appetite.
We were rarely able to find any kind of legitimate justification for products claiming they could suppress appetite, but Dr Collins suggests this might come from ingredients being higher in protein and fibre, which take longer to digest.
But he also warned: ‘You can't compensate for a bad diet by taking a supplement that's going to do that trick for you. However fantastic the claim is, it’s probably going to be of zero benefit, or the benefit will be so marginal it wouldn't be worth it.’
Some products included claims for ingredients which have been rejected and therefore shouldn't be used.
For example, 'Extreme Burn' supplements by Formula Max 5, found on eBay, claimed that raspberry fruit extract ‘assists the body to burn fat at a higher rate’ and that green coffee bean ‘decreases the absorption of carbohydrates’.
In reality, a slew of weight loss claims for raspberry extract have been rejected owing to a lack of compelling evidence, while the green coffee bean claim that it ‘acts by reducing the absorption of sugar (glucose) from the digestive tract’ has also been rejected.

Use our expert advice and recommendations to live your best life every day.
Get startedWe were also disappointed to see big-name retailers using suggestive terminology on their websites. Amazon, Holland & Barrett and Superdrug each had signposting on their websites for ‘fat burners’ and ‘appetite suppressants’.
These categories included supplements without any ingredients that justify these claims, and in some instances, there were no claims made by the product at all.
For example, at Holland & Barrett, the page and packaging for its Acai Berry tablets make no claims of any kind – because there are none that are authorised or on hold. However, if you go to the ‘fat burner’ section of the website, you’ll see the Acai product listed.
We feel this suggests to consumers effects the products won’t have.
Similarly, Superdrug’s ‘appetite suppressant’ section included a cinnamon supplement, which makes no claims to impact appetite. There are no approved or on-hold claims that cinnamon suppresses appetite.
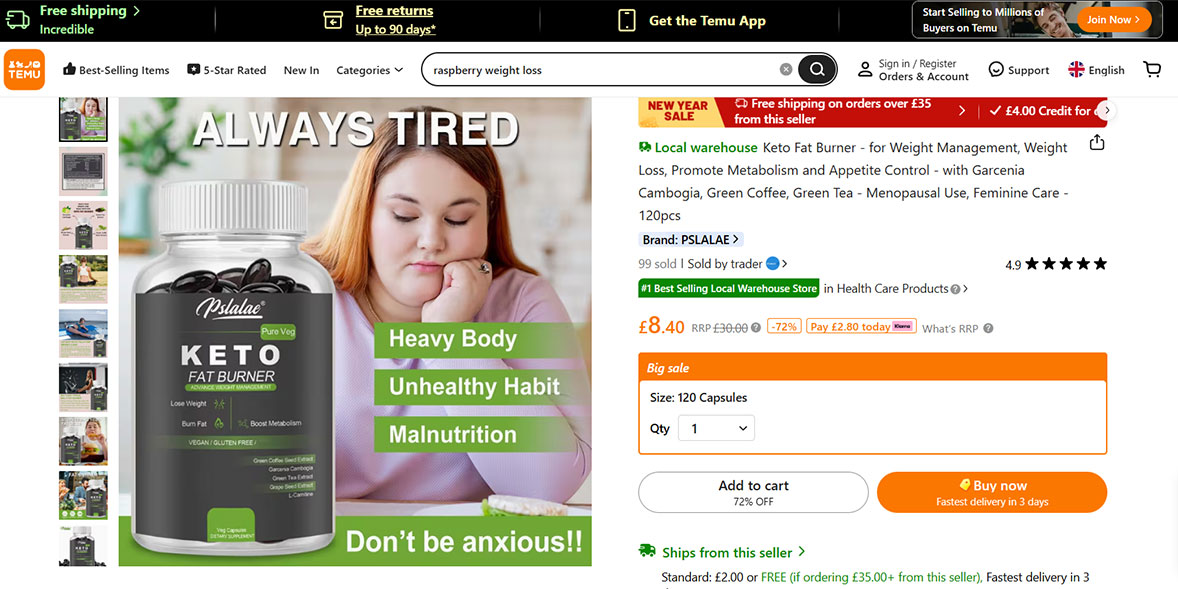
The Advertising Standards Authority (ASA) has ruled that products can’t say how much weight you’ll lose or how quickly, or from which part of your body – because this is something the brands simply don’t know.
But we’ve spotted plenty of claims that you’re going to 'lose weight fast', and that you’ll be able to target specific areas like your belly or arms.
For example, the 'keto fat burner' from the brand Pslalae on Temu claimed that you’d ‘lose your belly fat fast while you shred your stomach’, while the 'slimming capsule' from the brand Coolkin, also on Temu, claimed that you can ‘reduce waistline’ and ‘slim & tone stomach’.
Similarly, a product called ‘Gone Appetit’ from Internal Youth on Amazon suggests that within four weeks ‘you're losing centimetres from your waistline’ and within eight weeks ‘you're mentally free from food obsession’.
Increasingly, we're seeing supplements trying to position themselves as a 'natural alternative' to prescription-only weight loss injections and medications.
We found 'Phentramine 375', sold by Pharmaslim on eBay. This has a very similar name to the weight-loss drug phentermine (not authorised for use on the NHS, but available on private prescription) and the listing suggestively asks, ‘why use prescription weight loss pills when you can get non-prescription weight loss pills that have no negative side effects but are just as effective?’.
Internal Youth’s 'Gone Appetit', which we found for sale on Amazon, made a similar reference to prescription weight loss products, claiming it ‘supports natural GLP-1 production with clinically studied ingredients, helping you stay satisfied longer, curb cravings, and eat less — without Ozempic-like injections or harsh stimulants.’
This is not allowed. Food supplements are regulated by the Food Standards Agency, but if they make claims to treat or prevent disease, they are classified as a medicine and regulated by medical laws instead.
Lynda Scammell, head of borderline products at the Medicines Healthcare products Regulatory Agency (MHRA), said: ‘Any weight-loss product which is presented in a way that is typical of authorised medicines, or which has a product name which is similar to the name of a prescription medicine to the extent that it may cause confusion in the mind of the average UK citizen is not permitted.’
An ASA spokesperson added, ‘Our rules are clear that ads mustn’t make unauthorised health, medical or weight-loss claims. In particular, ads can’t claim or imply that a food supplement can provide effects associated with prescription-only weight loss medicines.'
Weight-loss jabs explained - get independent expert advice on the pros, cons, costs and experiences of members who've used them, and how weight loss pills compare
Sue Davies, Which? head of food policy said:

'It’s really worrying that online marketplaces and popular health retailers are promoting misleading health supplements.
'Not only does this make it impossible for shoppers to trust the claims they see online but it also means people could be wasting their hard-earned cash on products which just don’t live up to the claims.
'Better oversight of the industry is desperately needed so the government and regulators can crack down on these misleading listings and ensure that any sellers who break the rules are properly held to account.'
We shared our findings with the retailers and brands involved. This is what they said. Most took action as a result of our findings, with more than 55 listings removed, and some website categorisation amended.
To what extent retailers proactively review their products for these issues moving forward remains to be seen.
Amazon said, ‘We require all products offered in our store to comply with applicable laws, regulations and Amazon policies.
‘We develop innovative tools to prevent unsafe products from being listed. We continuously monitor our store, and we take action to maintain a safe selection for our customers, including removing noncompliant products, and outreach to sellers, manufacturers, and government agencies for additional information, when appropriate.
‘We have removed the highlighted products in question.’
eBay said, ‘Consumer safety is a top priority for eBay. We have reviewed the listings identified by Which? and have removed all items that are against eBay policy.
We use enforcement measures to help prevent unsafe items from being listed on eBay. These include seller compliance audits, block-filter algorithms, AI-supported monitoring by in-house specialists, and close partnerships with regulators. These measures help prevent millions of potentially unsafe items from being listed each year.’
Holland & Barrett said, ‘We are committed to providing high-quality, science-backed products that reflect the latest guidance. Product categorisation is intended to support customers to navigate our website, and we regularly carry out detailed reviews led by our science and regulatory teams to ensure this is consistent and helpful.
‘Following our latest review, the H&B Acai Berry tablets now sit within our superfood category, and the H&B Matcha Green Tea product can be found in the weight management category.’
Superdrug said, ‘Our customers’ health and wellbeing is always a priority. Superdrug Marketplace is a curated platform where third-party sellers must adhere to strict listing guidelines, including alignment with UK health authority recommendations. We do not intend to make unjustified health claims, and any categorisation on our website is designed to help customers navigate products rather than imply specific health outcomes.
‘Upon being made aware of Which?’s findings, we have paused all retailing of the highlighted product. We have also reviewed the category in question, and will take further action where necessary to ensure our content remains compliant and clear for customers.’
Temu said, ‘After receiving the inquiry, we immediately removed the products listed in the report pending further review and are working with the sellers involved to rectify their descriptions.
‘Temu maintains strict requirements for dietary supplements, requiring documentation such as HACCP certification and composition reports.
‘Following the ASA's advice on food supplements, Temu has been enforcing and will further enhance its review process. We are also providing additional compliance training to remind sellers of their obligations to meet the required regulatory standards.’
Coolkin said, 'Our products are certified before they are put on the shelves. There is no problem.'
Formula Max 5 did not respond to requests for comment.
Internal Youth said, ‘We have passed on your points to our marketing department who will be addressing each concern and actioning anything deemed inappropriate on our product listing immediately.’
Pharmaslim said, 'The product is manufactured in the UK in a licensed facility and is a food supplement, not a medicinal product. We do not make medical or therapeutic claims for it. For completeness, the listing you are referring to is not currently active, as the product is out of stock. We are reviewing the points you raise regarding product naming and marketing presentation.'
Pslalae did not respond to requests for comment.